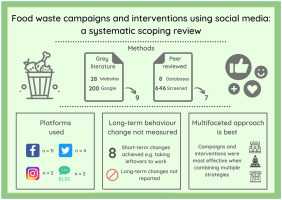
Begin by auditing the top 20 posts that promote non-evidence-based women’s health interventions and identify the exact claims, the promised effects, and the selling language. This upfront mapping provides practical help to teams to identify which messages drive engagement, who amplifies them, and what actions followers are urged to take, laying a solid groundwork for the protocol.
We apply participatory research methods to ensure diverse voices guide the analysis, especially african communities and women who have faced misinformation. Involve local partners such as claire and tamsenfadal in co-developing the coding framework, mapping not just what is said but why it resonates, and how it may create discomfort around legitimate care. Over years of work, we observe that jokes or light-hearted framing commonly mask serious claims, making critique harder but more persuasive, so our plans include unpacking tone and intent. Some posts frame risk claims as a joke to attract shares, which we flag in the coding.
We design a content analysis protocol with clear criteria for what counts as evidence, what constitutes performance claims, and what counts as selling or marketing tactics. The plans for data collection cover posts, visuals, cited sources, and comments threads. We measure effect on followers by tracking engagement rates, years of platform activity, and reportable outcomes, while experts review the coding decisions to improve reliability. The shih variable is used as a case example to illustrate how a single claim can propagate across platforms over years.
Beyond measurement, we emphasize the purpose of public health communication: to reduce harm from misinformation while supporting informed decision-making. We examine how misinformation may generate discomfort among audiences and how certain messages undermine trust in clinicians and researchers, including risk messaging about heart health. Our approach aims to produce actionable steps for researchers, educators, and platform teams to respond quickly and ethically.
Finally, we outline concrete recommendations for practice: publish a transparent methodology, share clear plans for community involvement, and provide practical guidance for content creators and platforms. We advocate for collaboration with community groups, experts, and health educators to curb risky selling tactics while promoting credible content that improves health literacy and outcomes. By documenting the pathways through which thegirlfrienddoctor and similar voices shape perception, we can build better safeguards and more accountable communications across years of activity.
Actionable Steps for Analyzing Menopause Content on Social Media
Define a precise sampling frame: specify population and platforms, including samples from Instagram, Facebook, TikTok, YouTube comments, and micro‑blog sites. Set a time window and language; specify initial inclusion criteria: posts that mention menopause, progesterone, cycles, intravaginal products, or related self‑care. The initial dataset should be screened for relevance, language quality, and geography to enable detection of differences across sources.
Collect and screen samples: draw samples flagged by keyword searches and hashtag collections; apply screening to exclude non‑menopause content or posts unrelated to health decisions. Screeners document reasons for exclusion; screened items feed into a log of samples with identifiers for interrater checks; provided metadata and links should be recorded, and you should note submitted elements that require verification.
Develop a coding framework: create categories to classify content into claim types (biomedical, lifestyle, self‑care), references to biomarkers, cycles, progesterone; include variables for post type, source cues, presence of disclaimers, and claimed outcomes. Ensure authors and community partners contribute to definitions; provide a detailed codebook with examples and explicit criteria for sign signals and differences across posts; types of content should be captured clearly.
Train coders and assess interrater reliability: conduct initial training with a subset of samples; use Byrd exemplars to calibrate coding; compute Cohen’s kappa or Krippendorff alpha; aim for agreement above 0.70 on key categories; discuss discrepancies and update the codebook; provided guidelines help ensure consistency across coders; require that all content in the sample be screened and re‑coded until stable.
Detect non‑evidence‑based claims: tag posts that assert biomarkers or cycles information without citations; flag mentions of intravaginal products or hormone therapies (including progesterone) claimed to alleviate menopause symptoms; capture whether claims are supported by sources or rely on anecdotes; note whether islam communities or other cultural contexts influence how claims are framed; track whether claims reach other posts or authors.
Incorporate consultation and cultural context: include a consultation with clinicians and community advisers to interpret context and avoid misinterpretation; submit the protocol for ethical review and ensure data privacy; when possible, plan to share a data dictionary and codebook with submitted results and provide feedback from authors; collect population input to refine categories.
Analyze and report findings: compute prevalence of different claim types, differences across platforms, and reach metrics; perform stratified analyses by post type and population; present results with clear visuals and a limitations note addressing sampling and coding biases; describe how products and interventions (including references to biomarkers) are portrayed in posts and what signals might indicate non‑evidence‑based messaging.
Documentation and governance: archive coding decisions, interrater decisions, and the initial version of the codebook; provide samples and quotes as de‑identified data; list authors and samples used in analysis; ensure that the submitted protocol and outputs remain transparent to readers and to the population studied, including islam communities when relevant.
Define Non-Evidence-Based Menopause Interventions: Criteria and Examples
Apply three criteria to define non-evidence-based menopause interventions: 1) no reliable scientific evidence from adequately powered trials; 2) claims built on testimonials, anecdotes, or marketing rather than data; 3) safety, dosage, and interaction details are missing or unclear. When a request is submitted by a patient, clinician, or online user, categorize the intervention for eligibility review if these gaps are present, and note the potential impacts on those seeking relief.
Examples (types) commonly seen include: herbal products claimed to balance hormones without consistent clinical data; mega-doses of vitamins or minerals with unclear benefit; detox diets and fasting regimens; detox teas; magnets or other energy devices; homeopathy; unregulated supplements; aromatherapy marketed for menopausal symptoms; and some acupuncture protocols where results are inconsistent. These items often target body symptoms and may promise power to change comfort levels, yet they lack robust scientific backing and safety profiles.
Assessment steps start with reviewing available scientific literature, regulatory status, and safety data; verify dosage information and any reported adverse events; evaluate potential interactions with prescribed therapy and existing body conditions. Determine reach and accessibility: those with lower income may face barriers to evidence-based care and might encounter cheaper, non-evidence options available online; four-fifths of commonly marketed items show gaps in evidence in some regions, highlighting concerns for patient safety and informed decision-making.
Future actions focus on clearer labeling, better communication with patients, and updates to clinician resources. Track indicators over weeks and quarters to see how uptake evolves and where gaps in understanding persist. For those shaping policy and education, align messages with topic-specific evidence, emphasize reliable sources, and provide practical guidance to help people choose safer options while reducing discomfort and misinformation in the world of menopause care.
Identify Platforms and Content Formats Used for Promotion
Prioritize video-first formats on the main channel set: Instagram Reels, TikTok, YouTube Shorts, and Facebook video posts. These formats are accessed by users with diverse interests and deliver quick, shareable messages. Produce 30–60 second clips with bold visuals, captions, and on-screen text to boost appeal. Test variations of hooks and calls to action. Track metrics such as reach, engagement, completion rate, and shares to locate where valuable attention appears and where it fades. Look for the sign of genuine engagement in comments and saved videos to guide next steps.
Address topics such as vaginal health, ovary, blood changes, and premenopausal symptoms. Distinguish what is believed by some audiences from what rests on evidence; when these claims are lacking support, add a clear disclaimer and point to reliable resources. Use explainer videos, text cards, carousels, and live Q&A sessions that show the claim, the basis, and a safe response. Ensure subtitles for accessibility and address transm discussions with careful language to reduce misinformation. Build a process to flag and correct misinformation quickly.
Platform risk management and audience fit: african communities are active on Facebook and WhatsApp; for discovery, prioritize Instagram and TikTok where younger audiences search for tips. Monitor comment sections for attacks and abuse; respond with factual corrections and provide resources. Use a channel-specific tone that respects privacy and protects those who share personal health concerns. Run A/B tests on formats to learn what resonates with different ages and interests while avoiding sensationalism.
Participatory design and safeguards: collaborate with community partners and researchers such as sonnenberg and harper to co-create content outlines and review claims. Involve women in african contexts to ensure topics, feel, and appeal align with real needs. Use surveys, comments, and feedback to refine content and reduce risk of harm. Establish clear moderation rules, accessible reporting, and a plan to address personal stories with care to protect those who disclose sensitive health experiences and those seeking information on transm discussions, such as vaginal health and premenstrual concerns.
Design a Participatory Data Collection Plan with Community Partners
Launch a six-week, co-designed data collection sprint with community partners, led by Lynne as the community liaison and Connie as the data lead, to prototype instruments, align ethics, and set shared goals before fieldwork begins.
- Stakeholder map and governance: Identify 6–8 partners across community health, workplace wellness programs, and local employers. Include at least one bank, a small/intermediate business, and a public health clinic in Mexico to ensure diverse usage contexts. Establish a joint steering group with biweekly 90-minute sessions, plus a 60-minute quarterly review to track process length and outcomes. Document roles, decision rules, and the agreement for data ownership and dissemination.
- Co-design of data instruments: Conduct two 90-minute workshops to co-create semi-structured interview guides, diaries, and photovoice prompts. Use real-world usage scenarios to surface concerns, pain points, and benefits. Capture items such as brand perceptions, hours of engagement, and potential flashes of insight that relate to non-evidence-based interventions. Produce instrument drafts within 10 days and finalize in week 4.
- Sampling and reach: Aim for a minimum of 40 participants, with 60% from workplace groups and 40% from community organizations. Track respondent characteristics by age, education, and lifespan stage to compute grouppercentage access and representation. Plan for accommodations to boost participation from underserved groups (e.g., flexible interview times, child care stipends).
- Ethics, consent, and agreements: Draft a concise consent form and data-handling agreement, signed by all partners before data collection. Include data use boundaries, retention length, and a clear path for withdrawal. Build in a community ethics review step with a local advisory panel and a dedicated “what’s new” update section for participants.
- Data collection methods: Combine diaries (two weeks, 1–2 pages daily), micro-surveys (monthly), and participatory interviews (weeks 3 and 6). Integrate photovoice to document lived experiences in workplace contexts and home life, including pain moments and moments of well-being. Ensure tools support multilingual usage and accessible formats.
- Logistics and resources: Schedule 2–hour collaborative sessions weekly, plus 1-hour check-ins for Lynne and Connie. Provide stipends or in-kind support (e.g., internet access, hours compensated) to sustain engagement. Leverage familiar platforms (e.g., Microsoft Teams) and offline options to maintain inclusivity across participants and environments (e.g., Mexico and other regions).
- Data quality and translation: Use dual coding with a rotating analyst pair, including at least one native speaker per language group. Perform back-translation checks on quotes and prompts. Maintain a living codebook accessible to all partners, updated after each data wave.
- Analysis plan and participatory synthesis: Host two 3-hour analysis workshops where partners review transcripts, map themes to concerns, and calculate a simple grouppercentage of identified priorities. Use consensus-building activities to generate actionable insights for campaign design, avoiding overinterpretation of non-evidence-based content.
- Dissemination and learning loops: Share findings in stakeholder briefings, community forums, and a concise, branded report. Promote transparent interpretation of benefits and limitations of interventions while highlighting practical recommendations for employers and community groups to increase safe engagement.
- Timeline and milestones:
- Week 0–1: establish governance, finalize agreement, recruit participants.
- Week 2–3: instrument co-design, pilot prompts with a small subset.
- Week 4: finalize data tools and consent documents, prepare data-logistics plan.
- Week 5–6: initiate data collection, mid-point check, begin coding.
- Week 7–12: continued collection, joint analysis sessions, draft dissemination plan.
- Expected benefits and risks: The participatory approach widens stakeholder buy-in, improves instrument relevance, and yields richer contextual data. Anticipate challenges with time commitments and language barriers; mitigate by providing flexible hours, multilingual staff, and clear expectations at every stage.
- Examples of concrete outputs: a field-tested diary template, a photovoice gallery with captions, a stakeholder-friendly data map, and a short evidence-informed guidance brief tailored for workplaces and community groups.
To scale impact, document each decision point, track use of the plan across forums, and celebrate early wins with small awards or recognitions for partners (e.g., an award for outstanding collaboration). Maintain a living ledger of lessons learned, including usage patterns, engagement hours, and the balance between promoted content and authentic participant voices.
Develop a Coding Scheme to Flag Unsubstantiated Claims and Misinformation
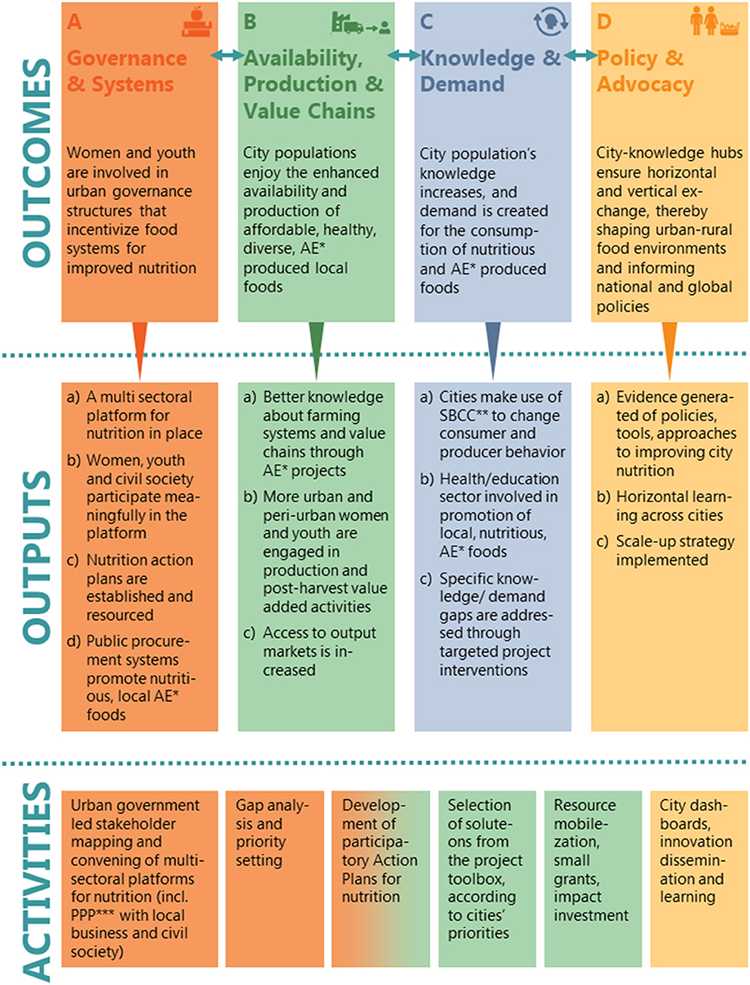
Implement a four‑tier coding scheme to flag unsubstantiated claims about interventions promoting women’s health. Start with a concise codebook that defines four codes: U1 Unsubstantiated efficacy claim; U2 Unsupported safety claim; U3 Anecdotal or narrative evidence; U4 Use of non‑credible sources. Include clear decision rules, example quotes, and fields for language, country, and platform. Use two coders per post and measure reliability with Cohen’s kappa, aiming for ≥0.70 on a 10% calibration sample. This approach supports participatory research by inviting aviva, elise, ghanem, knight to review the definitions and provide context on local usage in greece and beyond.
Indicators and decision rules. Distinguish proven from unproven claims by requiring credible citations (randomized controlled trials, systematic reviews, official guidelines) for effectiveness statements. Usually, posts that rely on testimonials, lifestyle anecdotes, or vague data without sources receive a U3 or U4 label. Indicators include phrases implying causation without data (eg, “caused by” a specific intervention) or broad generalizations (“most women”) without evidence. Use controlled data when comparisons are explicit, and flag posts that cite non‑peer sources or sensational media as U4. Maintain a range of evidence types across posts to avoid systematic bias in coding.
Coding structure and workflow. For each post, apply codes along four axes: Effectiveness, Safety, Evidence quality, and Source credibility. Tag each axis with the most salient code (U1–U4) and attach a short note explaining the reasoning. Capture comments from readers, noting whether the post relies on a narrative versus data, and compute the proportion of posts that merge narrative claims with data gaps. Use tech tools to tag keywords (eg, “proven,” “usually,” “tested,” “safety”) and to log decisions in a shared tract of notes. Analysing posts this way helps identify recurrence patterns and informs intervention design for lived experiences and lifestyle messaging.
Quality control and participatory practice. Conduct a calibration round with a panel that includes holders from community groups and researchers, such as aviva, elise, ghanem, and knight, to refine rules and address language nuances in greece and similar settings. After calibration, implement a double‑coding regime for 20–30% of the sample and resolve discrepancies through a adjudication log. Track intercoder reliability and adjust the codebook to improve reasonable agreement across languages and platforms. This collaborative step ensures the scheme respects local narrative forms while remaining rigorous against misinformation.
Reporting and output. Produce a table that links each post to codes U1–U4, with accompanying notes, country, platform, and a brief theme tag (eg, “interventions,” “skin,” or “lifestyle”). Report the proportion of posts flagged per theme and per country, and map high‑risk topics to content‑moderation or public‑education actions. Use the coding results to guide subsequent steps in the narrative analysis and to highlight gaps in evidence that may influence future interventions and policy discussions.
Construct Menopause FAQs from Community Inquiries and Verified Resources
Start by collecting three core questions from tina, sydney, and vanessa in community inquiries and map each to a verified medical resource; this, with clear and empowering language, boosts detection of accurate guidance and helps readers apply practical lifestyle tips, amplifying the power of community-informed answers and supports promotion of evidence-based information.
From several questions, readers want to know prevalence, typical length of symptoms, and what to do here instead of trusting unverified claims. Build on-demand posts that link to medical sources, include short quotes when helpful, and present a solution-focused path for readers seeking clarity. Admitting remaining gaps in evidence helps set realistic expectations. Readers having concerns about hormone therapy should consult a clinician.
To structure responses, center on three segments: detection and ovaries changes, testing and medical evaluation, and management options. Publish these on a home page and reserve space for updates as new evidence becomes available.
| FAQ Question | Verified Resource/Source | Practical Recommendation |
|---|---|---|
| What are common signs and how is detection determined? | Medical guidelines; clinician evaluation | Track frequency and duration of symptoms (hot flashes, sleep disruption); seek clinician confirmation; avoid self-diagnosis. |
| Which lifestyle steps have benefits and how to start? | Evidence-based lifestyle recommendations | Adopt regular physical activity, balanced meals, stress management; identify triggers; share plan with a care team. |
| When should I consider medical options, including oral therapies? | Guidance on safe therapies | Discuss risks and benefits with a clinician; some cases may use oral options with monitoring; never adjust medications without advice. |




